Onglyza & Kombiglyze XR Lawsuits – Heart Failure
 AstraZeneca’s prescription medication Onglyza (Saxagliptin), and Kombiglyze XR (Saxagliptin/Metormin), both brand name drugs used in the treatment of Type 2 diabetes, may cause an increase in certain cardiovascular events, and could even result in death. Onglyza received FDA approval on July 31st, 2009, and is used to help control high blood sugar in patients with Type 2 diabetes (who do not suffer from diabetic ketoacidosis). Kombiglyze XR was approved on November 5, 2010
AstraZeneca’s prescription medication Onglyza (Saxagliptin), and Kombiglyze XR (Saxagliptin/Metormin), both brand name drugs used in the treatment of Type 2 diabetes, may cause an increase in certain cardiovascular events, and could even result in death. Onglyza received FDA approval on July 31st, 2009, and is used to help control high blood sugar in patients with Type 2 diabetes (who do not suffer from diabetic ketoacidosis). Kombiglyze XR was approved on November 5, 2010
The Food and Drug Administration (FDA) has recently raised the alarm regarding potential harm caused by Onglyza and Kombiglyze XR – newer diabetes medications.
The FDA is investigating harmful effects caused by Onglyza and its sister medication Kombiglyze regarding an increased risk of heart failure. One of the FDA’s panel members advised that the drug should be withdrawn from the U.S. market but the safety panel opted to recommend a warning be added to updated prescribing information.
This is not the first problem that “newer” antidiabetic medications have presented and it is not the first sign of trouble for Onglyza. A 2013 study showed that medications that act in a similar fashion to Onglyza and Kombiglyze XR may also be linked to a higher than normal risk of pancreatic cancer.
Medications such as Onglyza and Kombiglyze XR are designed to improve the lives of patients who use them but in some cases, the medications end up causing more harm than good. If you or a loved one has suffered medical injury after taking Onglyza or Kombiglyze XR, you may be eligible for compensation.
Patients who have been injured by other antidiabetic medication have claimed that:
- The company manufactured a dangerous medication
- The company failed to adequately warn the medical community and the public about possible dangers
- The company improperly marketed medications
- The company knew about the dangers and chose to market the drug anyway
Patients or family members have been eligible in some cases for damages related to medical costs, lost wages, pain and suffering, wrongful death, and punitive damages. Each potential case of medical injury is unique and must be evaluated separately. Filing a lawsuit is no guarantee of success but if you or a family member has been injured by Onglyza, you should have your case evaluated by a legal expert.
The FDA Votes to Increase Warnings on Onglyza Labeling
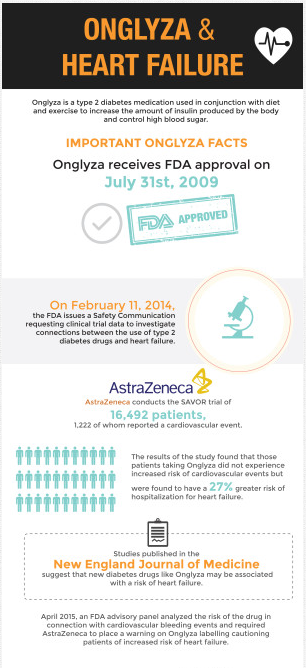 An FDA panel voted in April 2015 to require AstraZeneca to place a warning on Onglyza labeling which would caution patients of a possible increased risk of heart failure. This decision came after reviewing the data from the SAVOR study, which involved 16,492 patients, 1,222 of whom reported a major cardiovascular event. In this study, AstraZeneca was required to show that Onglyza would not increase the risk of heart failure or other cardiovascular conditions by more than 30 percent. It was found that those patients taking Onglyza were found to have a 27 percent greater risk of hospitalization for heart failure than those in the control group. Despite the manufacturer meeting the required threshold, the FDA panelists noted that more information was necessary in order to determine the actual risk of death from all causes associated with Onglyza. Indeed, fourteen of the fifteen panelists voted in favor of placing a warning on Onglyza labeling, while one panel member voted to withdraw Onglyza from the United States market altogether.
An FDA panel voted in April 2015 to require AstraZeneca to place a warning on Onglyza labeling which would caution patients of a possible increased risk of heart failure. This decision came after reviewing the data from the SAVOR study, which involved 16,492 patients, 1,222 of whom reported a major cardiovascular event. In this study, AstraZeneca was required to show that Onglyza would not increase the risk of heart failure or other cardiovascular conditions by more than 30 percent. It was found that those patients taking Onglyza were found to have a 27 percent greater risk of hospitalization for heart failure than those in the control group. Despite the manufacturer meeting the required threshold, the FDA panelists noted that more information was necessary in order to determine the actual risk of death from all causes associated with Onglyza. Indeed, fourteen of the fifteen panelists voted in favor of placing a warning on Onglyza labeling, while one panel member voted to withdraw Onglyza from the United States market altogether.
Symptoms of heart failure may include:
- Difficulty breathing
- Swelling of hands, feet or ankles
- Irregular heart rate
- Fatigue
- Persistent cough or wheezing
Getting the Help You Need
Patients with Type 2 diabetes can feel frustrated with their choices of drugs to treat their illness. If they take no drugs, they risk uncontrolled sugar levels, which bring a myriad of health complications, however some of the new diabetes drugs, such as Onglyza, may have serious and even fatal side effects. If you developed a heart failure after taking Onglyza, you may be eligible for financial compensation.
The Onglyza lawyers at McCorvey Law have the reputation and willingness to fight for our clients’ rights. If you suffered from heart failure after taking Onglyza, call our office today at 1-888-291-2431.
